POU
- Domain
- Homeobox|Pou
- Group
- Helix-turn-helix
- PFAM
- PF00157
- Desciption
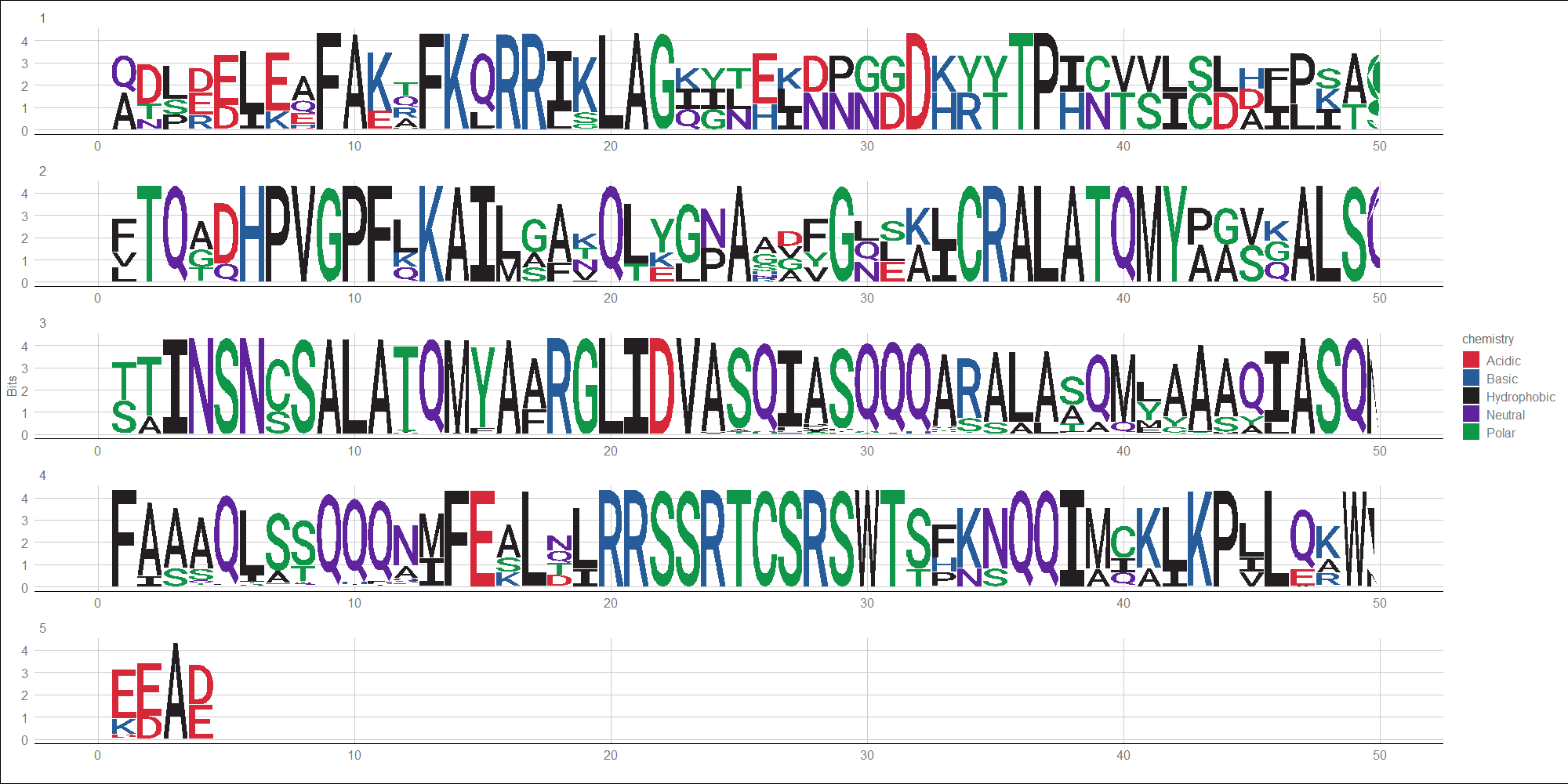
- The POU domain is a bipartite domain composed of two subunits separated by a non-conserved region of 15-55 aa. The N-terminal subunit is known as the POU-specific (POUs) domain (this entry), while the C-terminal subunit is a homeobox domain (IPR001356). Both subdomains contain the structural motif 'helix-turn-helix', which directly associates with the two components of bipartite DNA binding sites, and both are required for high affinity sequence-specific DNA-binding. 3D structures of complexes including both POU subdomains bound to DNA are available. The domain may also be involved in protein-protein interactions [6]. The subdomains are connected by a flexible linker [7, 5, 8]. Despite of the lack of sequence homology, the tridimensional structure of POUs is similar to 3D structure of bacteriophage lambda repressor and other members of HTH_3 family [7, 5]. POU proteins are eukaryotic transcription factors containing a bipartite DNA binding domain referred to as the POU domain. The acronym POU (pronounced 'pow') is derived from the names of three mammalian transcription factors, the pituitary-specific Pit-1, the octamer-binding proteins Oct-1 and Oct-2, and the neural Unc-86 from Caenorhabditis elegans. POU domain genes have been identified in diverse organisms including nematodes, flies, amphibians, fish and mammals but have not been yet identified in plants and fungi. The various members of the POU family have a wide variety of functions, all of which are related to the function of the neuroendocrine system [4] and the development of an organism [1]. Some other genes are also regulated, including those for immunoglobulin light and heavy chains (Oct-2) [3, 2], and trophic hormone genes, such as those for prolactin and growth hormone (Pit-1).
- Rederence
-
- POU domain factors in the neuroendocrine system: lessons from developmental biology provide insights into human disease. Andersen B, Rosenfeld MG. Endocr. Rev. 22, 2-35, (2001).
- Binding of a Drosophila POU-domain protein to a sequence element regulating gene expression in specific dopaminergic neurons. Johnson WA, Hirsh J. Nature 343, 467-70, (1990).
- Characterization of chicken octamer-binding proteins demonstrates that POU domain-containing homeobox transcription factors have been highly conserved during vertebrate evolution. Petryniak B, Staudt LM, Postema CE, McCormack WT, Thompson CB. Proc. Natl. Acad. Sci. U.S.A. 87, 1099-103, (1990).
- The solution structure of the Oct-1 POU-specific domain reveals a striking similarity to the bacteriophage lambda repressor DNA-binding domain. Assa-Munt N, Mortishire-Smith RJ, Aurora R, Herr W, Wright PE. Cell 73, 193-205, (1993).
- Crystal structure of the Oct-1 POU domain bound to an octamer site: DNA recognition with tethered DNA-binding modules. Klemm JD, Rould MA, Aurora R, Herr W, Pabo CO. Cell 77, 21-32, (1994).
- Brain 4: a novel mammalian POU domain transcription factor exhibiting restricted brain-specific expression. Mathis JM, Simmons DM, He X, Swanson LW, Rosenfeld MG. EMBO J. 11, 2551-61, (1992).
- The virtuoso of versatility: POU proteins that flex to fit. Phillips K, Luisi B. J. Mol. Biol. 302, 1023-39, (2000).
- Structure of Pit-1 POU domain bound to DNA as a dimer: unexpected arrangement and flexibility. Jacobson EM, Li P, Leon-del-Rio A, Rosenfeld MG, Aggarwal AK. Genes Dev. 11, 198-212, (1997).
Statistic of Transcription Factors
DNA Logo
Download
Transcription Factor Table
| Gene ID | Insect | Gene Name | Gene Symbol |
|---|---|---|---|
| iTF_00000309 | Abrostola tripartita | Atri018077.1 | SGF3 |
| iTF_00000310 | Abrostola tripartita | Atri020812.1 | nub |
| iTF_00000311 | Abrostola tripartita | Atri004317.1 | acj6 |
| iTF_00000312 | Abrostola tripartita | Atri012468.1 | POU6F2 |